- Tritium Periodic Table
- Tritium Atomic Number 1
- Tritium Atomic Number Mass
- Tritium Element Atomic Number
- Atomic Number Of Tritium
Tritium, a radioisotope of hydrogen with the gross atomic mass of 3.014, is considered an important and versatile radioisotope among twenty five hundred radioisotopes discovered mainly by nuclear transmutation reactions during the last sixty years. A radioisotope can be depicted by its atomic number or chemical symbol and by its mass number that indicates the total number of protons and neutrons in the nucleus of the radioisotope. Thus tritium can be depicted as hydrogen-3. Together with two other stable isotopes of hydrogen (hydrogen-1 and hydrogen-2), and with isotopes of carbon, nitrogen, oxygen, phosphorous and sulfur, tritium provides a powerful tool for understanding chemical, biological and geochemical transformations. Tritium has also found widespread uses as a tracer in medicine, agriculture and industry.
- Tritium (H-3) has an atomic number of 1 and a half-life of 12.7 years. It is a low energy 0.019 Mev (max) beta emitter. The emitted beta radiation cannot penetrate the outer layer of dead skin. There is no external exposure hazard and no required shielding.
- Tritium, (T, or 3 H), the isotope of hydrogen with atomic weight of approximately 3. Its nucleus, consisting of one proton and two neutrons, has triple the mass of the nucleus of ordinary hydrogen. Tritium is a radioactive species having a half-life of 12.32 years; it occurs in natural water with an abundance of 10 -18 of that of natural hydrogen.
The discovery of tritium in 1932 involved the work of several very eminent scientists that included Lord Rutherford, Sir John Cockroft, Ernest Lawrence, Luis Alvarez, Willard Libby--just to name a few. The story of the discovery of tritium is worth telling for it shows that even the most outstanding and prolific physicist of this century, Lord Rutherford, made an error of judgment and thus was not credited for discovering tritium.

Tritium has an atomic mass of 3.0160492 u. Diatomic tritium (T 2 or 3 H 2) is a gas at standard temperature and pressure. Combined with oxygen, it forms a liquid called tritiated water (T 2 O). Tritium's specific activity is 9,650 curies per gram (3.57 × 10 14 Bq/g).
Following the discovery of stable hydrogen-2 (commonly called deuterium), by Harold Urey, the method for the isolation of deuterium oxide or heavy water from natural water by electrolytic process made it possible to prepare deuterium gas. With the availability of accelerators like Lawrence's cyclotron or Cockroft and Walton's machine, it became possible to produce radioisotopes by bombarding elements with a beam of accelerated ions like protons, deuterons or alpha particles (helium ions). In one of his last scientific experiments, Lord Rutherford bombarded heavy water with a beam of deuterons accelerated by the Cockroft and Walton machine. After a careful examination of the products of reaction (the hallmark of the Cambridge Laboratory in UK), it was revealed that two near nuclear species with mass number 3 could be identified i.e., one was tritium (H-3) and the other helium-3 (He-3) lighter than ordinary helium (He-4).
With the availability of deuteron beam from Ernest Lawrence's cyclotron at the University of California, Berkeley, new isotopes were being produced by bombarding anything the physicists could get hold of. This was to study scattering of particles produced in the bombardments, but the researchers did not use deuterium oxide (heavy water).
In discussions with Ernest Lawrence, Joliot-Curie reported that he had been successful in producing a radioisotope of phosphorus by bombarding aluminum with alpha particles from a polonium source. The radioisotope decayed with a half life of about 2.5 minutes with the emission of positrons. It is said that on receiving this news from Joliot-Curie, Ernest Lawrence immediately went to the Berkeley laboratory and discovered substantial amount of radioisotope of sodium there. As a matter of fact, it was causing some health hazard to workers in the laboratory. Obviously, Joliot-Curie was credited for the discovery of artificial radioactivity, although large number of radioisotopes had been produced in the Berkeley laboratory but never recognized before.
It is interesting to find mistakes made by very eminent scientists during their career. Not only Rutherford and Lawrence made mistakes, even Fermi thought that fission products produced in the irradiation of uranium with neutrons were transuranics and not fission products. One finds numerous examples if one looks up the history of science.
Irradiation of deuterium oxide was never attempted in the Berkeley Laboratory since Rutherford had already identified protons and neutrons resulting from collision of deuterons with deuterium oxide that, presumably, produced tritium and helium-3. Rutherford realized that of these two nuclear species, one must be stable. He made the mistake by considering tritium as the stable species and helium-3 as the radioactive one. He pursued this idea with some vigour. With his influence, he persuaded the Norsk Hydro (Norwegian) heavy water plant to concentrate tritium oxide by the electrolytic process from 99.99% heavy water. He then asked F.W. Aston, the inventor of the mass spectrometer, to analyze the sample with the most sensitive techniques. Tritium was not recorded although the technique was capable of detecting two parts of tritium in one hundred thousand parts of deuterium. Measurements conducted almost twenty years later show that tritium was present to the extent of one part per million in the sample.
A bright, young physicist, Luis Alvarez at Berkeley, after hearing of Rutherford's work, realized Rutherford's mistake in regarding tritium as being stable. His failure to find tritium in the concentrate from Norsk Hydro could be attributed to the fact that tritium was radioactive and that helium-3 was stable. Luis Alvarez, a very versatile physicist whom I met during my graduate studies at Berkeley and who has now been credited with many outstanding discoveries, proceeded to convert a cyclotron into a mass spectrometer dedicated to determine species with mass number 3. He found helium-3 in helium gas from oil wells as well as helium from the atmosphere. He concluded that helium-3 was either stable or it had a very long half life. The residence time of the gas in oil wells had to be very long since a radioisotope of relatively short half life will not survive in the sample. He then proceeded to prepare tritium by bombarding a lithium target with neutrons. It was simple to isolate tritium from the target by reacting lithium with water. The radioactivity from hydrogen gas along with molecular species with tritium atoms thus isolated, was established with an ionization chamber. Samples prepared in 1935, and left behind in the Cambridge laboratory by Rutherford) were analyzed with a large volume Geiger counter in 1949. Radioassay of the sample permitted the evaluation of the sample and allowed an estimate of the half life of tritium as approximately 12 years. Later research has refined this figure. We now know that the radioisotope decays with the half life of 12.35 years by the emission of ß- (beta-minus or electron) to helium-3 with end-point energy of beta particles as 18.6 keV (kilo electron-volts). It took almost twenty years to characterize this radioisotope. In the mean time, it was established that tritium was continually being produced by nuclear transmutations brought about by cosmic-ray neutrons with air in much the same way as carbon-14 is produced in atmosphere. It is brought down to the earth along with rain water. The sample for Rutherford had tritium produced by cosmic-ray neutrons. Fresh rain water contains the highest concentration of tritium.
W.F. Libby pioneered the method of establishing the age of vintage wine. For example, twenty-year old wine is expected to have tritium content one third of the amount found in fresh rain water, or a twenty-five old wine would show one fourth of the activity found in fresh water. Thus, this method of dating water has found extensive applications in hydrology as well.
The methods of large scale production of tritium are now well established. Irradiation of lithium-6 with neutrons in a nuclear reactor results in the formation of tritium and helium-4. After World War II, tritium was manufactured on a very large scale for assembling thermonuclear devices. Lithium deuteride and tritide are placed along with a conventional atomic bomb. Deuterium and tritium react with each other at very high temperatures to form helium-4 and a neutron. A nuclear transmutation reaction of a neutron with lithium-6 produces more tritium. The overall reaction involving lithium and deuterium produces two helium-4 atoms. It is hoped that in the not too distant future, electricity will be generated by controlled thermonuclear process involving deuterium and tritium. At present tritium is also being produced as a by-product in nuclear power reactors in Canada as a by-product. Neutron capture by deuterium in heavy water results in the formation of tritium, and Ontario Hydro has a large inventory of tritium in their possession.
Tritium is one of the least expensive radioisotopes and this makes large-scale tracer experiments possible. The half life of 12.35 years of the isotope is conveniently long for tracer studies and its availability in highly specific activities also makes it possible to conduct biological tracer experiments. Large numbers of compounds labeled with tritium are commercially available.
Tritium use can be classified under four categories, namely, physical, chemical, biological and clinical medicine. Under the physical category, tritium is used as a target material for generating fast neutrons, or as a source of Bremsstrahlung radiation (as in light sources)* for the preparation of luminous compounds. Weapons manufacture can be also mentioned. Under the chemical category, tritium is used in hydrogen transfer reactions or in analytical chemistry as a tracer. In biology it is used in investigating metabolic pathways, in cytological applications, in the assay of enzymes and in hydrogen transfer studies. Lastly in clinical medicine, tritium is used for diagnostic, pharmaceutical and radiotherapeutic purposes.
It can be seen that this radioisotope of element number one in the periodic table of elements, (the first radioisotope in the periodic table of elements), provided quite a challenge in its discovery and in characterizing its properties. It holds a great promise for the future of mankind for it may become an important component in generating electricity by its use in controlled thermonuclear reactors. Tritium's present uses in research, industry, hydrology, biology are too many to catalogue in this article and it is certain that is applications in various human endeavor will grow in the future.
*(Ed Note:) It might be of interest to note that substantial tritium contamination occurs in landfills from discarded wrist watches which have been crushed by machines grading the fill areas.
Tritium Periodic Table
Also found in: Thesaurus, Medical, Financial, Encyclopedia, Wikipedia.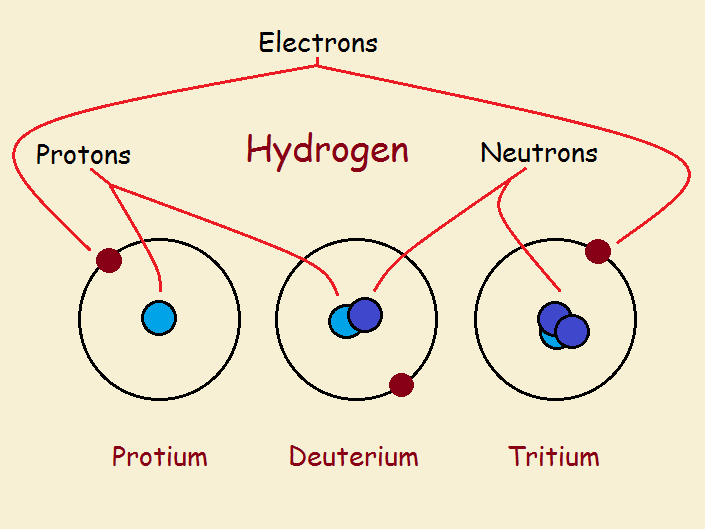
trit·i·um
(trĭt′ē-əm, trĭsh′ē-)n.tritium
 n
n
trit•i•um
(ˈtrɪt i əm, ˈtrɪʃ-, ˈtrɪʃ əm)n.
trit·i·um
Tritium Atomic Number 1
(trĭt′ē-əm, trĭsh′ē-əm)tritium
| Noun | 1. | tritium - a radioactive isotope of hydrogen; atoms of tritium have three times the mass of ordinary hydrogen atoms atomic number 1, H, hydrogen - a nonmetallic univalent element that is normally a colorless and odorless highly flammable diatomic gas; the simplest and lightest and most abundant element in the universe |
Tritium Atomic Number Mass
tritium
Tritium Element Atomic Number
[ˈtrɪtɪəm]N → tritiomtritium
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:
Atomic Number Of Tritium
